Lunac: the rare combination of ultimate hardness and corrosion resistance
as well as cathodic protection capacity in the case of Lunac 2+ duplex
Almost no affordable construction metal is capable to combine good corrosion resistance and high hardness / high tensile strength. Noble metals are generally too soft to withstand any significant (adhesive or abrasive) wear load. High tensile or hardened steel does not offer sufficient corrosion resistance to withstand moderate up to high corrosive conditions. Consequently, these two important |
characteristics for many applications require special solutions to be acquired simultaneously. Coatings can offer the solution. However, almost any coating which is hard as well as corrosion resistant suffers from cracking, porosity or bond problems. We solved this wide spread limitation successfully after a long research program with the hard, corrosion resistant and crack-free Lunac coatings. |
Corrosion testing:
In the diagrams below you can find corrosion levels of various materials in a strong
halogen HCl/HF acid mixture (representative for many harsh corrosive conditions).
Note, strong oxidizing agents such as HNO3 can often
practice a protective effect
on chromium or high chromium containing steel.
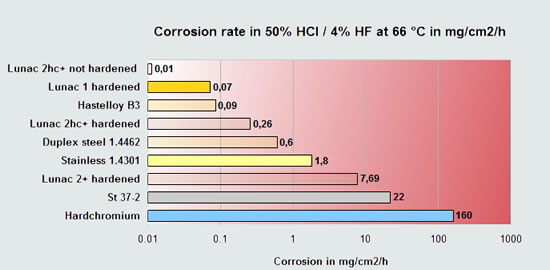
Diagram 1
In corrosion perspective this corrosion testing diagram displays two different metal types : |
Metals with improved resistance to oxidizing environment such as HNO3 or H2O2. These metals can benefit from the metal oxide augmenting action of these chemicals, provided that the metal oxide layer is dense and offers good bonding. Chromium, Titanium, Stainless steel or Aluminum are good examples of these metals or alloys. Lunac coatings, copper or steel do not comply with this mechanism and are less chemically resistant in these chemicals. |
Metals with improved resistance to non-oxidizing acidic environments such as HCl, H2SO4, HF, acetic acid, citric acid etc. Therefore, the majority of acids. These metals and alloys mostly benefit from their higher electro-potential, so the more noble base metals and noble metals. Copper, Tin, Nickel, Lunac 1 and 2+ or silver are good examples of these metals or alloys. The metals as mentioned at 1. show visa versa no good corrosion resistance to these acids. |
Corrosion mechanisms:
Both hard and corrosion resistant Lunac 1 and 2+ industrial coatings stay fully crack- and pore-free in the case the substrate is 1- free of serious defects and 2- ground smoothly in advance (Rz< 2.0 µm / 0.079 mil). The latest introduced technical hard nickel intermediate coating offers ultimate repair capability of surface micro-defects. Good corrosion resistance can not only be obtained by higher electro-potentials or dense oxide layers, but also by means of stress-free lattices, or even better, amorphous structures. In this way a material or coating with a lower electro-potential but crystal free |
structure can often deliver the same corrosion resistance as a more noble crystalline material. The additional advantage of such system is less galvanic corrosive activity and substantial cost savings. There are Lunac versions with no crystalline structure and small potential differences with steel. Moreover, hard coatings such as the Lunac systems which contains crack inhibiting compressive stresses are very rare.
The latest Lunac 2+ duplex version even resists corrosion after plastic deformations because of the ductility of the intermediate technical hard nickel and the superb bond quality. |

Figure 1: The consequences of porosity in a HVOF ceramic coating on 1.4462 (duplex steel) with high pitting and inter granular corrosion resistance, became visible not before the ceramic coating was removed from this prop shaft. The corrosion beneath the coating reached out over +/- 4 mm depth. Most likely the steel suffered from this corrosion because of a devastating salt concentration effect and high local temperatures, despite the application of the highly sea water resistant Chromium-Nickel-Molybdenum steel. Lunac coatings proved to inhibit this effect due to the dense structure, good heat conductance and their inertness.
Figure 2: Lunac 1 (displayed left) and Lunac 2+ coatings contain compressive stresses and will consequently inhibit micro-crack formation during hardening. Hard chromium coatings (displayed below) develop micro-cracks after being deposited, due to tensile stresses. These micro-cracks generally highly compromise the corrosion resistance.


Figure 3: Submersed sea water exposure test of smoothly ground St 52.3 rods, plated with 50 µm Lunac 2+, not hardened (left) and hardened (right). Start of tests: 06/10/07 and 01/04/08. The corrosion resistance of the hardened Lunac 2+ version is reduced, but still good enough to perform well in sea water. This offers hardened Lunac 2+ the unique feature of being very hard (HRc 72/Hv 2200) as well as corrosion resistant as well as crack free. For these characteristics Lunac 2+(duplex) is very often applied to hydraulics and axles in marine environments. The surface of Lunac 2+ coatings can slowly tarnish when not used in the same way copper does (the surface of the rod in the right bottle turned a bit gray. After a few days the slightest pore would have commenced the production of massive orange 'rust clouds'. Date of photo: December the 29th , 2011.

WMV corrosion tester for Neutral and Acidic Salt Spray Testing.
Duplex Lunac coatings are able to seal steel surfaces
completely and comply with the ASTM NSS B117, 1000
hour salt spray testing with rating 10 (no single rust spot).
The standard Lunac 2+ coating dissolves with 0.21 µm/year
in sea
water at 20°C (68 F). This offers a theoretical > 100
years
service life in this perspective.
Corrosion and sub surface corrosion of coated carbon steel in a locally
destructed coating or
coating end zone will significantly be reduced
by stress-free and cathodic protective Lunac 2+ duplex coatings compared
to
almost any other hard coating system
In a corrosive environment almost all protective noble (metallic) coatings or stressed coatings (such as hard chromium !) will accelerate the steel (galvanic) substrate corrosion in damaged of boundary spots. As a reference, |
cathodic coatings such as zinc or aluminum show a protective behaviour. Lunac 2+ duplex coatings are optimised to come very close to these protective coatings and will not show significant sub-surface corrosion either. |

Corrosion test rods with 3.0 holes after 37° C / 99°F , 400 hours 4% salt spray corrosion testing before cleaning.
Left: 30/30 µm duplex chromium on carbon steel with a 3 mm/.12" hole (originally). The (sub surface) corrosive product emission after 8 days acidic salt water corrosion testing is clearly visible.
Right: 120 µm Lunac 2+ duplex on carbon steel with a 3.0 mm/.12" hole. No sub surface corrosion can be observed and the corrosive product emission after 8 days acidic salt water corrosion testing is highly reduced. The (in depth) galvanic corrosion is one of the lowest achievable in the category 'sea water resistant coating'.
Hard chromium on carbon steel (picture after cleaning)
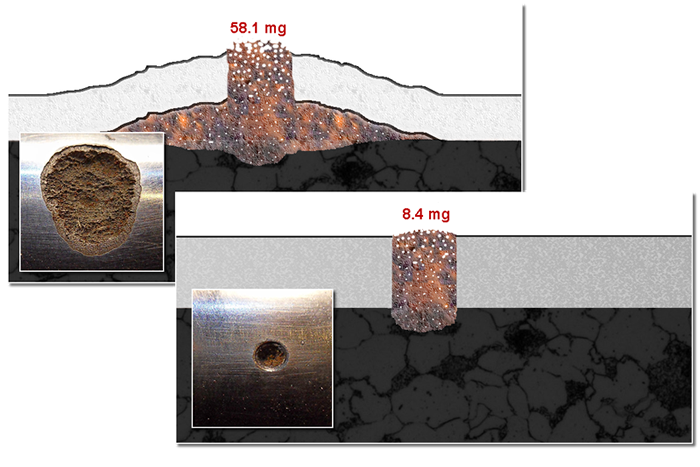
Lunac 2+ duplex on carbon steel (picture after cleaning)
1000 hours NSS corrosion testings in Ø 3.0 mm / .12" (800 µm / 0.03" deep) 'through coating' holes at 37°C / 99°F.
show the amount of corrosion after the first 8 days salt water corrosion testing. Note: the 30/30 µm chromium coating develops an additional, hardly soluble iron oxide layer (visualized in brown) with major sub surface spreading characteristics.
Lunac 2+ duplex is one of the rare hard coatings
that is able to inhibit sub-surface corrosion as well
Neutral salt spray tests in the case of
moderate
(up to 70 µm) indentations

Corrosion after indentation
Although most Nickel-chromium coatings on carbon steel perform well within 1000 hours of Neutral Salt Spray testing and are capable the deal with small accidents as well, they generally start developing rust spots after the 1000 hours period. All tested hard and brittle mono-layer coatings show up corrosion along the micro-cracked edges (picture 6 and 7). Only the Lunac 2+ duplex coating proved to be a very long lasting hard corrosion-protective coating (2400 hours NSS test already proven) that is capable to deal with small indentations as well.
Lunac 2+ Grayish appearance
- The grayish appearance of the Lunac 2+ surface after prolonged water contact is caused by the development of a +/- 0.2 µm / 0.0079 mil stable conversion layer.
Comparison HVOF ceramic, hard chromium and Lunac 2+
- In contrast to HVOF ceramic duplex coatings, Lunac 2+ duplex does never peel and Ni-Cr should not peel on loading.
In perspective of hardness and scratch resistance, Lunac 2+ should be compared with HVOF ceramic coatings.
Lunac 2+ duplex has since the 26th of September 2011
been qualified to offer long lasting corrosion protection,
according to NBD 10300 (Rijkswaterstaat). This test is
based on the
EPQ test as described in ASTM
G59-97
(2003)
and ASTM G61-86 (2003).

Test performed by Independent testing institute C-Cube
